What are the options for large scale hydrogen energy storage?
Sept 30, 2022
Hydrogen is one of the buzzwords of the future of energy and is sometimes described as a silver bullet in energy (though mostly by people who don’t really know anything about hydrogen…). This is because it has a number of very attractive properties: It can be made by electrolysing water, of which we have an almost unlimited resource, and then when it is burned (or reacted in a fuel cell) there is no CO2 emission associated with power generation - the main product is again water. It also has a very high energy density by weight - 1kg of has a Lower Heating Value (LHV - the amount of energy available from combustion) of 33.33 kWh/kg (compared to gasoline with a LHV of 12.34 kWh/kg). In this post, I am not going to dwell on the pros and cons of ‘green hydrogen’ (spoiler - there are a lot of cons associated with hydrogen production by electrolysis which mean it is not entirely obvious whether we should be spending money on hydrogen as a serious option for energy and transport in future) - that is hydrogen made from zero carbon electricity, but I want to focus at the ways in which we may be able to store hydrogen.
There are three main ways that have been proposed for hydrogen storage so far:
1. Hydrogen liquid (LH2)
2. Compressed H2 gas
3. Material based hydrogen storage
Liquid Hydrogen
To make liquid hydrogen (denoted LH2) requires very cold temperatures and, for the liquid state to exist, H2 must be cooled below its critical point (a temperature of 33K). At atmospheric pressure, this temperature is even lower - it must be cooled to ~20K. LH2 is attractive as a fuel since it does not require very high pressures for storage. However, the energy density by volume is still much lower than standard fuels, such as gasoline, since the density of LH2 is low (70.85 kg/m3 at 20 K). This gives a volumetric energy density of 2361 kWh/m3 for LH2 as compared to 8675 kWh/kg for Octane.
The main challenge with the storage of LH2 are the energy requirements to cool it down sufficiently. The minimum energy requirements to liquefy H2 from ambient conditions are 3.9 kWh/kg (with conversion to para-LH2 which is standard practice). Actual liquefaction requirements are substantially higher, with current best practice achieving 10-13 kWh/kg, though some research suggests that this could be reduced to 7 kWh/kg. Thus the energy for liquefaction is likely to represent at least 20 percent of the LHV for liquid hydrogen.
LH2 is typically stored at temperatures around 23K and pressures of 2-4 bar. To maintain the temperature at that level requires a constant boil off, maintaining the liquid state by evaporative cooling. This is a significant loss in small containers, however in larger well-insulated containers this loss can be made quite small. Boil off losses can be reduced to less than 1% in tanks with volumes above 50 m3.
Compressed Hydrogen
Hydrogen is a gas at normal atmospheric pressures and temperatures with a density of 0.84 kg/m3. It is this very low density that means it is a challenge to store and most applications store highly compressed hydrogen gas. For example, hydrogen storage tanks for hydrogen powered cars contain hydrogen at pressures up to 700 bar (the density of H2 at 700bar is 42 kg/m3 giving a volumetric energy density of 1400 kWh/m3). This compression of hydrogen requires energy, just like the cooling to make hydrogen liquid. The theoretical minimum energy required to compress Hydrogen isothermally from 20 bar up to 700 bar (a typical pressure at which an electrolyser might work) is 1.36 kWh/kg. If H2 were generated at ambient conditions an additional minimum of 1.05 kWh/kg would be required for isothermal compression. In practice, considerably more energy is required for this, estimated around 6 kWh/kg H2. Assuming compression work of 3 kWh/kg is achievable, compression work would account for 9% of the LHV.
The big challenge with storing hydrogen at high pressure is the need for high tensile strength materials. Often, composite materials are used and making the tanks lightweight is very difficult, this is why rockets use liquid hydrogen. In hydrogen vehicles, carbon fibre tanks with metal liners are used, with additional overwrap to withstand the enormous pressures. The gravimetric efficiency of pressurised Hydrogen also decreases as the pressure increases - i.e. the ratio of the weight of the hydrogen to the weight of the container decreases with pressure. We can see this If we consider a cylinder (neglecting the ends). The cylinder stress is: \begin{equation} \sigma_{cy} = \frac{pr}{\tau} \end{equation}
Where $\tau$ is the wall thickness, $p$ is the pressure and $r$ is the radius. The mass of the tank material is the volume of the material $V_{mat}$ multiplied by the density $\rho_{mat}$, therefore: \begin{equation} m_{mat} = 2 \pi r L \tau \rho_{mat} \end{equation}
The mass of hydrogen contained is the tank volume times the density: \begin{equation} m_{H2} = \pi r^2 L \rho_{H2} \end{equation}
Combining these equations gives the following expression: \begin{equation} \frac{m_{H2}}{m_{mat}} = \frac{\sigma_{cy} \rho_{H2}}{2 p \rho_{mat}} \end{equation}
Since the compressibility increases with pressure, then this ratio decreases with pressure, and thus more material is required for H2 storage at high pressures. This is a major challenge for applications such as aerospace, and means that you cannot simply add more H2 fuel without adding proportionally more weight. Figure 2 shows the proportion of hydrogen by weight when contained in a steel pressure vessel with an allowable cylinder stress of 500MPa.
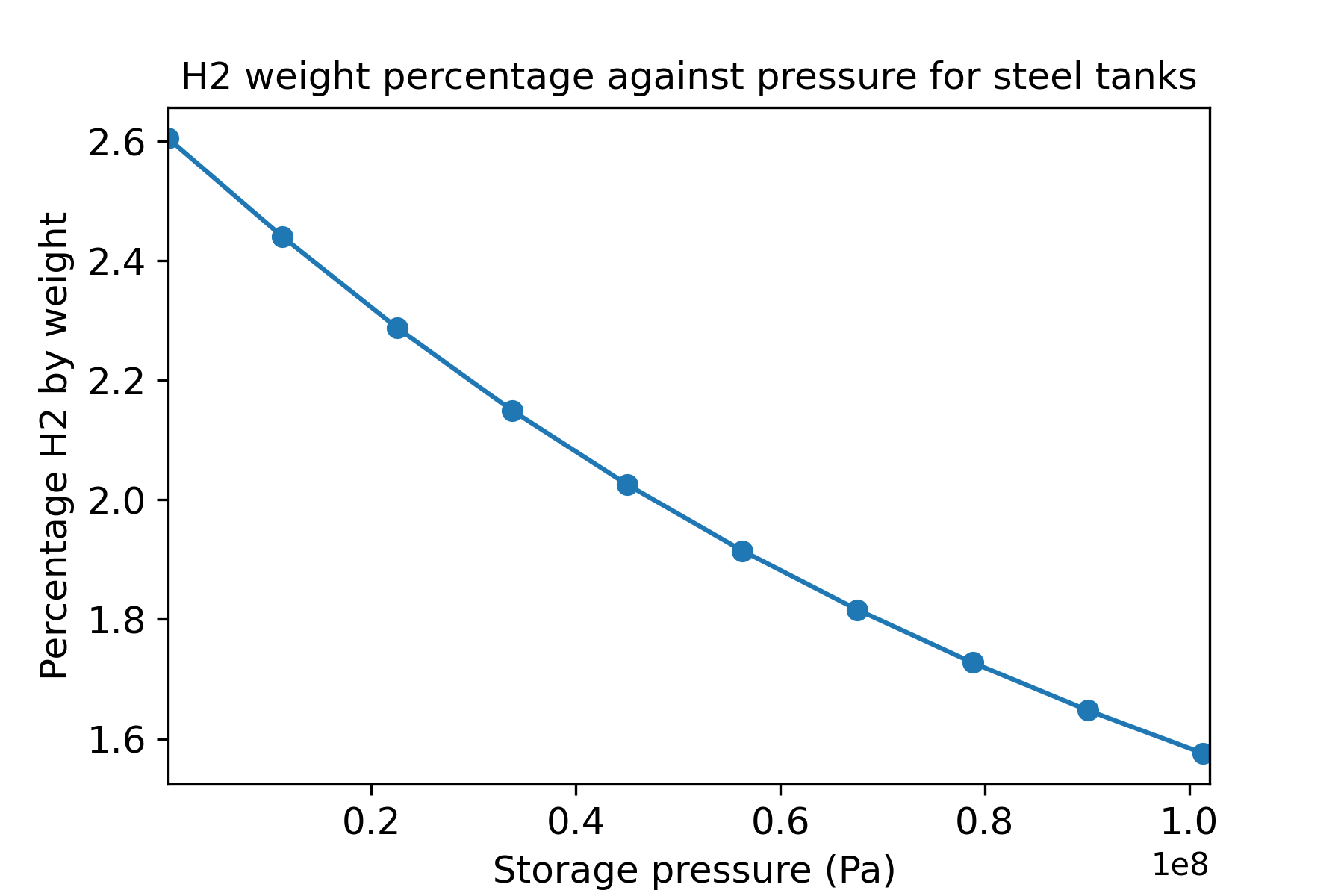
Figure 1: Hydrogen weight percentage assuming a steel cylinder with allowable cylinder stress of 500 MPa.
The alternative to high pressure storage is low pressure storage of H2 gas. This has potential advantages of lower costs and relies on the ability to have large footprints and low-cost materials which are largely impermeable to Hydrogen. The most famous example of low pressure Hydrogen storage also illustrates another hugely important(!) aspect of Hydrogen storage, which we won’t discuss here, and that’s safety. The example, of course, is the Hindenburg airship, which used Hydrogen not as a fuel but as a method of achieving buoyancy in air. This used a membrane of Goldbeater’s skin (treated cow’s stomach) to contain Hydrogen used to float the airship. More modern approaches to low pressure H2 storage propose using plastics, such as High Density Polyethylene (HDPE), possibly with an inner liner to reduce hydrogen permeability.
Pressurised H2 can also be stored in underground salt caverns, and there are a number of demonstration projects aiming to test viability. These include a three-cavern system in Teeside, UK and three facilities in TX, USA.
Material Storage
The final method is to store the hydrogen by either using chemical bonding to materials or by adsorption to materials. Metal hydrides are one of the most commonly suggested groups of materials due to the fact that they offer Hydrogen storage at standard temperatures and moderate pressures. The main challenges relate to cost, cyclability, lowering operating temperatures and increasing the hydrogen storage by weight (gravimetric efficiency). Liquid Organic Hydrogen Carriers (LOHCs) are also promising options. Hydrogen is stored by chemically bonding with hydrogen-lean molecules and it is released through a catalytic dehydrogenation. LOHCs offer potential for easy management in ambient conditions, and the store and release processes are carbon free and the carrier liquid is not consumed. So far the storage capacity is the limiting factor.
In physical sorption, hydrogen binds to the surfaces of materials and thus highly porous materials, with large surface areas are attractive for Hydrogen storage. Porous carbon materials have been proposed and Metal Organic Frameworks (MOFs) are another highly porous material that appear promising. Typically this method of Hydrogen storage requires low temperature and high pressure, with the research mainly focussing on increasing hydrogen storage capacity at standard conditions. Figure 2 shows how Hydrogen may be stored in a MOF with applications in the automotive industry.
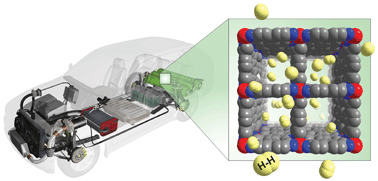
Illustrating how hydrogen can be stored on the surface of metal organic frameworks. Hydrogen bonds to the surface and is stored within the materials pores. Image credit.
Summary
While there are a number of promising research directions, there remain challenges with all the aforementioned hydrogen storage methods. At present, compressed H2 storage is leading and benefits from the fact that we already distribute compressed natural gas and have experienced working with various other pressurised fluids. However, finding a lightweight material that is sufficiently cheap to make this method a widespread option is still a way off. At large scale, hydrogen storage in salt caverns seems plausible and is a leading contender. What process will win out in the long run for large-scale hydrogen storage, if any, remains uncertain.