Flow Batteries
Batteries with external electrolytes.
(Redox) Flow Batteries (RFB) are a form of a battery in which the electrolyte contains one or more dissolved electro-active species which flow through a power cell/reactor where the chemical energy is converted to electricity. They are based on the reduction-oxidation reaction between two electrolytes. The reaction is reversible allowing the battery to be charged, discharged and recharged. The electrolytes are stored in external tanks and pumped through separate circuits for positive and negative species, while an ion exchange membrane separates them within the reaction chamber. The cell voltage is determined by the Nernst equation, and is typically between 1 and 2.2V. As the power is determined by the size of the reactor while the energy capacity is determined by the physical size of the storage tanks, the power density is decoupled from the energy density in a RFB.
Flow batteries can release energy continuously at a high rate of discharge for long durations. They also have no self-discharge as there is no reaction outside of the reaction chamber. Unlike with fuel cells where only the electro-active chemicals (e.g. hydrogen, methanol, and oxygen) flow through the reactor and the electrolyte remains at all times within the reactor, flow batteries drive (both) the electrolyte flows through the reactor. As the chemical reaction in flow batteries is reversible, just like conventional electrochemical batteries they can be recharged without replacing the electro-active material. One attraction of flow batteries to EV applications is the ability to “recharge” them by simply replacing the electrolyte akin to filling up the fuel tank for an internal combustion engine.
There are three different electrolytes that form the basis of the existing designs of flow batteries currently in demonstration or in large-scale project development.
Vanadium Redox Batteries (VRB)
VRB batteries exploit the ability of Vanadium to exist in four different oxidation states. In each cell of a VRB, the vanadium redox couples are stored in a mild sulfuric acid electrolyte. The vanadium couples are V2+/V3+ in the negative and V4+/V5+ in the positive half-cells. During the charge/discharge cycles, H+ ions are exchanged between the two electrolyte tanks through a hydrogen-ion permeable polymer-membrane, producing a voltage of 1.4–1.6 V. The reactions can be expressed as:
Positive Electrode: V4+ → V5+ + e- (discharging)
Negative Electrode: V3+ + e- → V2+ (discharging)
The efficiency of this battery is generally considered around 85%. VRB’s have several potential applications in enhanced power quality, UPS, peak shaving, increased security of supply and integration with renewable energy systems. The majority of development work has focused on stationary applications due to the relatively low energy density. Estimating the costs of VRB is difficult as it depends in part on the number of annual charge/discharge cycles.
Work has recently begun on the world's largest vanadium flow battery in Dalian China, a 200MW/800MWh monster [1].
Zinc Bromine batteries (ZRB)
In a Zn-Br battery, two different electrolytes flow past carbon–plastic composite electrodes in two compartments separated by a micro-porous polyolefin membrane. During discharge, Zn and Br combine into zinc bromide, generating 1.8V across each cell. During charge, metallic zinc will be deposited (plated) as a thin film on one side of the carbon–plastic composite electrode. The net efficiency of this battery is about 75%. The reactions are:
Positive Electrode: Zn → Zn2+ + 2e- (discharging)
Negative Electrode: Br2 + 2e- → 2Br+ (discharging)
Zn-Br batteries offer the high cell voltages of flow batteries and two electrons are released per atom of Zinc. This gives them the highest energy density among currently available flow batteries.
Zn-Br batteries have also enjoyed significant development and are available as residential storage systems from a number of manufacturers. For example, the 10kWh Redflow ZCell is available for around $20,000 AUD including the installation and inverter.
Polysulphide Bromide (PSB)
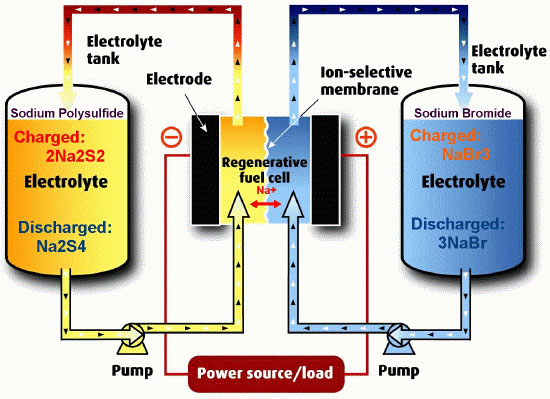
Figure: A depiction of a PSB flow battery
PSB is a regenerative fuel cell technology that provides a reversible electrochemical reaction between two salt solution electrolytes (sodium bromide and sodium polysulphide). PSB electrolytes are brought close together in the reactor cells where they are separated by a polymer membrane that only allows sodium ions to go through, producing about 1.5V. The net efficiency of this battery is about 75%. In the bromine/polysulfide system, the positive electrolyte is sodium bromide, and the negative electrolyte is sodium polysulfide. At the positive electrode, three bromide ions combine to form a tribromide ion. At the negative electrode, the sulfur in solution is shuttled between polysulfide and sulphide. The reactions are:
Positive Electrode: Br2 + 2e- → 2Br2- (discharging)
Negative Electrode: 2S2 → S42- + 2e- (discharging)
PSB has been verified in the laboratory and demonstrated at multi-kW scale in the UK. The key attributes of this system are that the species that comprise the two electrolytes are abundant and reasonably inexpensive. Furthermore, they are highly soluble in aqueous electrolytes meaning that the volume of electrolyte required to store a given quantity of charge is reduced. In 2002 a 15MW 120MWh Regenesys PSB flow battery was built at Little Barford in the UK. The exact details of the project are scarce but the project was never fully commissioned. The business was owned by RWE power who pulled out before commissioning leaving the project with nowhere to go. RWE stated that they believed the technology would be viable but was still a few years from being truly commercial.
Organic Flow-Batteries
In 2013 researchers announced the use of 9,10-anthraquinone-2,7-disulphonic acid (AQDS), a quinone, as a charge carrier in metal-free flow batteries [2]. AQDS undergoes extremely rapid and reversible two-electron two-proton reduction on a glassy carbon electrode in sulphuric acid. Each of the carbon-based molecules holds two units of electrical charge, compared with one unit in conventional batteries, implying that an organic flow battery could store twice as much energy as a conventional flow battery in a given volume. This is a promising research area as it has the potential to offer a low-cost flow battery electrolyte.
Summary of characteristics
| Type | Typical Capacity | Typical Power | Efficiency (%) | Storage Duration | $/kWh | $/kW | Lifespan (years) | Cycling capacity |
| VRB | Up to 40MWh | Up to 20MW | 75-80 | hours-weeks | ~200 [3] | ~1000 [3] | 25+ | 10000+ |
| Zn-Br | Up to 3MWh | Up to 500kW | 75-80 | hours-months | ~400 | ~40 [4] | 20 | 3000 |
| PSB | Up to 120MWh | Up to 15MW | 75 [5] | hours-months | 150-1000 [5] | 700-2500 [5] | 5-30+ | 3000 |
Table: Main flow battery chemistries and typical characteristics
References
[1] New generation of ‘flow batteries’ could eventually sustain a grid powered by the sun and wind