Batteries
A booming industry
There are many different types of electrochemical battery technologies available for energy storage. Batteries that involve multiple charging/discharging cycles are also sometimes referred to as secondary (re-chargeable) batteries, rather than primary batteries which are designed to be used once and then disposed.
Battery technologies are currently the most widespread and satisfactory methods of storing relatively small amounts of energy for powering portable electrical devices. Several variants have also been used for grid applications, especially for power quality, Uninterruptible Power Supply (UPS) and spinning reserve. Recently, there has also been significant deployment of batteries for energy management, especially at a domestic level. Batteries are potentially well suited to applications at this scale due to their compact size and modularity. The Tesla Powerwall is just one of a number of increasingly popular battery options.
An electrochemical battery contains a negative and a positive activation species, with different battery variants using different activation species. During charging the positive active species is oxidised, while the negative is reduced. There are a wide range of battery technologies available.
Lead-Acid batteries
Lead-acid batteries are commercially mature re-chargeable batteries. They are supplied by a large, well-established, supply chain and have the largest market share for rechargeable batteries both in terms of sales value and MWh of production, with major contributions coming from the automotive industry and industrial batteries for standby applications.
In terms of chemistry they generally consist of lead metal and lead dioxide electrodes immersed in a sulphuric acid electrolyte (in the charged state). Both electrodes are converted to lead sulphate during discharge and the concentration of the sulphuric acid electrolyte is reduced as it becomes largely water. The nominal cell voltage is relatively high at 2.05 V. The reactions are as follows:
Positive Electrode: Pb + HSO4- →PbSO4 + H+ + 2e- (discharge)
Negative Electrode: PbO2 + HSO4- + 3H+ → PbSO4 + 2H2O (discharge)
Lead-acid batteries are commonly used in stationary energy storage applications, especially as a DC auxiliary. Large lead acid batteries of 1-5MW have also have an established history in submarine use. They are suitable for power quality, UPS and spinning reserve applications. Notable lead-acid battery installations include a 40 MWh system in Chino, California, with a rated power of 10 MW for 4 hours. There is also over 100MW of battery power capacity currently installed for standby duty throughout the National Power and PowerGen power companies in the United Kingdom [1].
The drawbacks of lead-acid batteries are primarily to do with low cycling capacity, high charge time and careful maintenance requirements coming largely from the evolution of H2 and water loss. This renders them largely unsuitable for energy management applications. They also have a low energy density to weight ratio (currently around 40 Wh/kg), due to the high density of lead.
Current research effort is targeted at the inclusion of carbon into the negative plate of the battery to improve the peak power handling capacity of the cells, reduce the need for maintenance and improve the deep discharge capability and cycle life of the technology.
Nickel based batteries (Ni-Cd, Ni-MH, NaNiCl2 etc.)
Nickel-Cadmium batteries
There are several nickel battery variants. Nickel-Cadmium batteries are the most mature of the Nickel variants. NiCd batteries contain a nickel hydroxide positive electrode, a cadmium hydroxide negative electrode, a separator, and an alkaline electrolyte, most commonly KOH. They usually have a metal case with a sealing plate equipped with a self-sealing safety valve.
The reactions are as follows:
Positive Electrode: 2NiOOH + H2O → 2Ni(OH)2 + OH- (discharge)
Negative Electrode: Cd + 2OH- → Cd(OH)2 + 2e- (discharge)
Ni-Cd batteries have been used in some utility-scale applications, notably the 14,000 cell Ni-Cd storage system installation run by GVEA in Fairbanks, Alaska which is one of the world’s most powerful battery. The 6.5 MWh can discharge at 46MW for 5 min. Ni-Cd batteries usually have slightly higher energy density than lead-acid types (around 50Wh/kg), can tolerate a deep state of discharge for relatively long periods, and require less maintenance than lead-acid batteries. Their cycling ability is a little higher than lead-acid but still relatively low (up to 2000 cycles). Although they require less maintenance than lead-acid, they require careful management to avoid the “memory effect” and to avoid over-charging. There are safety and environmental waste issues with Ni-Cd batteries because Cadmium is a very toxic substance. They have largely been superseded by NiMH battery variants.
Nickel Metal Hydride batteries
Nickel Metal Hydride (NiMH) batteries have largely replaced Ni-Cd batteries for use in portable re-chargeable batteries. Developed in the 90’s these batteries have a significantly higher energy density than Ni-Cd batteries (around 80 Wh/kg), have less environmental issues and are cheaper. However their main disadvantages are high self-discharge rates and a relatively low cycling capacity. They have been used in hybrid vehicles.
Sodium Nickel Chloride batteries
The sodium-nickel chloride battery is better known as ZEBRA battery. It is a high temperature system, operating around 300C, much like the sodium sulphur battery, however it has a lower energy density (around 100 Wh/kg) and specific power (around 150W/kg) [2]. The negative electrode is made of molten sodium while the positive electrode is nickel in the discharged state and nickel chloride in the charged state. The electrolyte is a molten sodium salt.
The reactions are as follows:
Positive Electrode: NiCl2 + 2Na+ + 2e- → Ni + 2NaCl (discharge)
Negative Electrode: 2Na → 2Na+ + 2e- (discharge)
Recently there has been a growing interest in the NaNiCl2 batteries for grid and EV energy storage applications, and these batteries are being developed and manufactured by General Electric (after acquiring Beta R&D in 2007) and Fiamm SoNick. NaNiCl2 batteries have a much lower self-discharge rate than and better cycling capabilities than the other Nickel battery variants. Accordingly they show potential promise for stationary grid energy storage applications as well as for electric vehicles.
Lithium-Ion Batteries
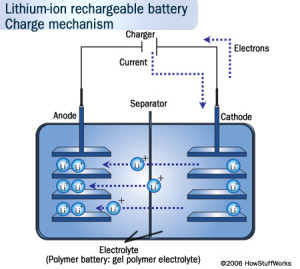
Lithium ion batteries are now the dominant type of batteries found in small portable electronic applications due to their high energy density, light-weight and high efficiencies. The negative electrode in these batteries is a lithiated metal oxide ((LiCoO2, LiMO2, LiNiO2) and the positive electrode is made of graphitic carbon with a layered structure. Electrolytes generally consist of lithium salts dissolved in organic carbonates, i.e. LiPF6 in ethylene carbonate. When the battery is being charged, the lithium atoms in the positive electrode become ions and migrate through the electrolyte toward the negative carbon electrode where they combine with external electrons and are deposited between carbon layers as lithium atoms. Discharging reverses the process. Examples of the reactions that occur in a LiCoO2 and LiC6 battery are:
Positive Electrode: CoO2 + Li+ + e- → LiCoO2 (discharge)
Negative Electrode: LiC6 → C6 + Li+ + e- (discharge)
These batteries have high energy densities (in excess of 150Wh/kg), high efficiency, a low rate of self-discharge (around 5% per month) and a good cycle life provided they aren’t fully discharged.
Figure: Illustrating the charge mechanism for a Li-ion battery (borrowed from howstuffworks).
Lithium ion batteries are set to be the dominant battery for the electric vehicle market, and their development by leading EV manufacturers like Tesla has driven their costs down. Their costs have now fallen to the point that there is significant interest in Lithium ion for grid energy storage. Elon Musk promised that he could deliver the South Australian grid the world's biggest battery in less than 100 days, and Tesla subsequently built the Hornsdale Energy Reserve near Adeliade, South Australia. This battery is used to help integrate wind power from a neighbouring wind farm into the grid and ensure relaibility. It has a storage capacity of 129MWh using 12,500 battery pods and can deliver 100MW of power, responding in 0.5secs. Larger battery storage schemes are planned, for example Florida Power & Light are planning the Manatee Energy Storage Center, which will have a power of 400MW and a storage capacity of 900 MWh.
Sodium-Sulphur Batteries
Sodium Sulphur (NaS) batteries are high temperature molten metal batteries. The appeal with these batteries is largely due to the scarcity of Lithium, however this has not yet reached the point where industry is particularly concerned. The negative electrode is made of liquid sodium while the electrolyte is solid beta-aluminium (a type of aluminium oxide). The positive electrode is molten sulphur. In order to keep the electrodes in their liquid states the system must be regulated at around 300oC. The solid electrolyte is known as a BASE (Beta-Aluminium Solid Electrolyte) and it selectively conducts sodium ions.
During discharge, the sodium atom in the negative electrode gives up an electron and migrates to the positive electrode. Here an electron reacts with the Sulphur and then combines with the sodium to form sodium polysulphide. The process is reversible and charging causes the polysulphide to release the electrons and the sodium ions migrate back through the electrolyte to recombine with the electrons at the negative electrode forming elemental sodium. The discharge reaction is as follows:
2Na + 4S → Na2S4 (discharge)
Once running the high temperature is self-sustaining from the exothermic reactions, and when the battery is cooled self-discharge cannot occur as no chemical reactions can take place. However, maintaining the temperature while the battery is neither charging or discharging is a form of energy loss.
Due to the temperature requirements these type of cells become more economical with bigger size as the volume-to-surface-area ratio reduces. They have a very small self-discharge because the electrolyte is a very poor conductor of electrons.
NaS batteries have high efficiencies (85-92%) and high energy and power densities (150-240 Wh/kg and 150-230 W/kg) [2]. There are more than 316 MW installed globally at 221 sites, representing 1896 MWh of installed storage capacity [3]. They are most suited to stationary grid applications due to high operating temperatures and corrosive nature of sodium polysulphide. Safety is also an issue as elemental sodium will ignite in contact with air or moisture.
NaS batteries have been used in grid-scale applications, for example, the 34-megawatt, 245-megawatt-hour hybrid wind and storage system in Aomori, Japan. However, interest in these batteries has waned due to their high costs and chequered historical safety record. A prominent fire at one of NGK's NaS systems is one example.
Metal-air batteries
Metal-air batteries are unique because one of the reactants (the air) doesn’t have to be stored in the battery and hence this type of battery has a very high specific energy density. Zn-air, Li-air and Al-air variants have all attracted attention, and many other metals can be used. Metal-air batteries consist of an exposed porous carbon electrode (called the air cathode) which is separated from the metal anode by an electrolyte. The exposed carbon electrode traps oxygen atoms from the air which react with the positive metal ions from the anode. Research into solid, liquid, aqueous and organic electrolytes has been undertaken but at present the non-aqueous electrolyte is the most developed. The reaction for the non-aqueous Lithium-air battery is as follows:
2Li + O2 → Li2O2 (discharge)
The main advantage of this type of battery is the huge increase in energy density over more conventional batteries; for example, a Zn-air has a theoretical energy density of 1353 Wh/kg [4]. However, this is a technology firmly in Research and Development and at present metal-air batteries have poor efficiency and cycling capability. Due to their potentially huge increase in specific energy density, EVs are one of the primary applications for metal-air batteries, and companies such as Tesla have some interest.
Summary of Battery characteristics
| Type | Typical Capacity | Typical Power | Efficiency (%) | Storage Duration | $/kWh | Lifespan | Cycling capacity |
| Pb-A | Up to 40MWh | Up to 20MW | 70-90 [2] | Seconds – days | 150-200 [5] | Up to 15 years | 1000-3000 cycles (max 50% DOD) |
| Ni based | Up to 20 MWh | Up to 50MW | 72-78 [7] | Seconds-days | 200-600 [7] (NiCd), 100-200 [2] (NaNiCl2) | Up to 20 years | 1500-3000 |
| Li-ion | Up to 100MW | 90-95 | Seconds - hours | 500-1000 [5,6] | Up to 15 years | 5000 cycles at 80% D.O.D. | |
| Na-S | Up to 250MWh | Up to 50MW | 75-85 [8] | Seconds - hours | 290-350 [8] | 5-15 years | 3000 cycles 100% D.O.D. |
Table: Common electrochemical battery chemistries and characteristics
References
[1] Ter-Gazarian, A., 2011. Energy Storage for Power Systems, s.l.: IET Power and Energy Series.
[3] EPRI, 2010. Electricity Energy Storage Technology Options
[6] Revealed: True cost of Tesla big battery, and its government contract